Endangered species stem cells: Emerging hope to save a species
We all need hope, but sometimes it is hard to see the distinction between hope and reality. Hope galvanizes our resolve as we make progress, no matter how miniscule the advance.
Previously, I wrote about the hope of modern science to use resources preserved in the Frozen Zoo® to help save the northern white rhinoceros. I want to share with you my first experiences that gave me hope for saving species through stem cells. Stem cells are special because they have the ability to give rise to any cell type in the body. Here at the Institute for Conservation Research, we hope to harness that ability to generate gametes, and by doing so save a species from extinction.
As part of my degree, I was a CIRM (California Institute of Regenerative Medicine) intern in Dr. Jeanne Loring’s lab when the stem cell research for the Northern White Rhino Initiative began. I was excited to work with my mentors, Cullen Pivaroff and Suzanne Peterson, at the Scripps Research Institute on any type of stem cell research, including their Parkinson’s research. It was quite a surprise to find out that the San Diego Zoo needed stem cells, especially on rhinos, and I couldn’t imagine why. Disease research and treatments… or something else?
Cullen surprised me by explaining that the stem cells were hopefully going to be used to make sperm and eggs for in vitro fertilization and embryo transfer to a surrogate mother. With only three northern white rhinos left at the time (and now only two) the population can’t reproduce naturally. If we can create stem cells, then in the future we can use them for assisted reproduction and save the species.
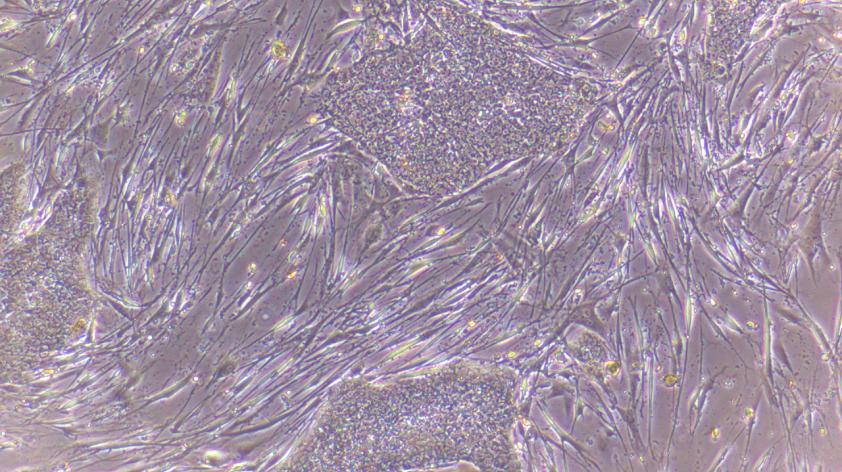
Cullen and I started the reprogramming process of converting skin cells into stem cells using a virus. The next day, we were excited to see that the rhino skin cells survived the viral reprogramming treatment.
Most reprogramming methods are toxic to the cells to a degree and there was only the slightest hope of success. It takes weeks for reprogramming to sway the cells from their fibroblast cell behavior into stem cells. We checked daily for signs of emerging stem cell colonies or cell death. Initially we were happy that most of the rhino cells survived the reprogramming.
Our feelings changed as the cells continued to grow and divide. We worried they would outgrow their tiny culture dish and the experiment would end without producing a colony of stem cells.
A few weeks later, as he peered through the microscope at the dish of overgrown rhino cells, Cullen decided to pull the plug on the experiment. I was disappointed that the experiment had not yielded a stem cell colony.
Over the course of those few weeks, I had learned about the plan featured in Zoo Biology to save the northern white rhino using a variety of artificial reproductive technologies, including in vitro fertilization. I believed this experiment was more than just hope; it was destined to be a part of that plan.
I peered one last time into the microscope, hoping to see a distinct border between the dense growth of skin cells and an emerging colony of stem cells. It was like trying to see through the impenetrable flurry of a blizzard, searching for the outline of a snow covered…anything. I did not see any distinct borders or any consistent flat layer of closely packed cells, the hallmarks of a stem cell colony.
The edge of the dish was an optical nightmare, creating a curvature that was hard to focus on. But… was the distorted focus on the edge of the plate camouflaging a tightly packed flat layer of cells? Were the angles obscuring the distinct border between the overgrown cells and a colony of stem cells growing onto the side of the dish? The stem cell colony was only visible by tilting the plate! Could this be something that might allow humanity to redress our impact on the northern white rhino and help rewind their extinction?
“Is this a colony?” I blurted out excitedly.
He squinted while tilting the plate under the microscope. His intense scrutiny revealed his excitement, but his scientific training only allowed a reserved skepticism. The colony was hard to see, but Cullen determined that it was a stem cell colony just emerging.
Under the microscope, he surgically removed the colony using a needle’s edge as a scalpel to lift and transfer it from the overgrown plate into a new spacious one. It would take a few more weeks before we could really say if the reprogramming to stem cells was successful, but until then we had hope. When that colony stabilized into a robust stem cell line, I realized that persistence could turn hope into reality.
That first colony of Angalifu’s stem cells from Dr. Loring’s lab gives hope that one day he will father offspring.
Angalifu, Nola, Dinka, Saut, and Suni, were all northern white rhinos that died before they could reproduce. Their fibroblasts, and now their stem cells created at San Diego Zoo’s Institute for Conservation Research, persevere in the Frozen Zoo® as hope for their species’ survival and as ambition that humans can make amends for forcing them to the edge of extinction.
We have made many advances in rhino stem cell science since my first experience in 2016, but those are stories for another time.













